Vulnerable Targets for Contraception in the Female
Authors
INTRODUCTION
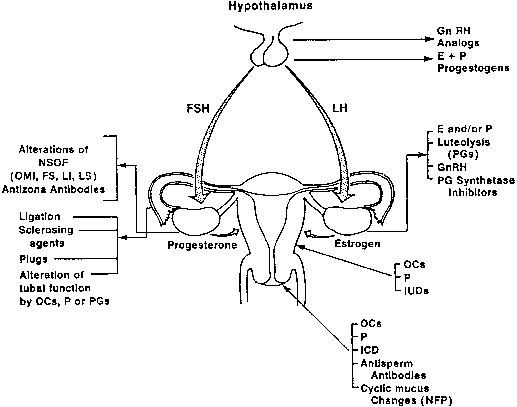
Development of new contraceptives for the female depends on a thorough knowledge of the anatomy and physiology of the reproductive tract. The female has five major roles in reproduction: (1) developing a gamete (ovum); (2) receiving the male gametes (spermatozoa) and transporting them from the vagina to the distal portion of the oviducts; (3) supplying a suitable milieu for fertilization; (4) directing the fertilized ovum across the fallopian tubes to the uterine cavity; and (5) providing a uterine environment conducive to implantation and maintenance of gestation. These functions provide an excellent opportunity for biologic and pharmacologic manipulation of the female reproductive process to achieve fertility control. This chapter reviews potentially vulnerable sites in the complex reproductive events in the female (Fig. 1).
HYPOTHALAMIC–PITUITARY REGULATION
Various studies have greatly clarified the interrelationship of the hypothalamic–pituitary axis. It is now well established that the hypothalamic decapeptide gonadotropin-releasing hormone (GnRH), responsible for the release of the gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH), must reach the anterior pituitary in episodic pulses approximately every 90 minutes to elicit a normal response. The secreted GnRH has a very short half-life (2–4 minutes), allowing for a rapid decay of the stimulus. Identification and synthesis of GnRH and its analogues have prompted the use of these agents for suppression of endogenous FSH and LH. Studies by Hotchkiss and Knobil of subhuman primates,1 and confirmatory reports in humans, have clearly shown that continued administration of GnRH or its superagonists leads to an initial phase of stimulation of pituitary gonadotropin release, followed by a return to baseline levels or below.2 Frequent pulsatile administration of GnRH can eventually shut down pituitary gonadotropin release, resulting in transient chemical castration. Receptor-binding studies have shown that the effect is owing to a decrease in the number of GnRH receptors on the pituitary gonadotropes, not to an alteration in the affinity of the receptors to GnRH.
Large numbers of reports on experimental animals have documented that both agonistic and antagonistic GnRH peptides are potent inhibitors of the reproductive process. Depending on the species used, dosage given, and mode and frequency of administration, these peptides have produced paradoxical effects, such as anovulation, luteal-phase deficiency, and termination of established pregnancy.3, 4
Gonadotropin-Releasing Hormone Analogues
Extensive clinical studies have been performed to test the contraceptive potential of GnRH agonists/antagonists with a variety of dosage regimens. There appears to be a great deal of individual variation to a given dose of this agent. With daily therapy at sufficiently high doses, complete ovarian inhibition, anovulation, and amenorrhea can be obtained. With progressively lower doses, lesser effects, characterized by oligo-ovulation and a clinical syndrome similar to dysfunctional uterine bleeding, may be observed. The clinical spectrum of induced ovarian insufficiency produced by these agonistic peptides, depending on dosage and duration of use, may range from total amenorrhea approximating castration to infrequent periods, to acyclic bleeding, to intermittent and unpredictable ovulation, to anovulation. A potential problem to be resolved lies in careful regulation of ovarian suppression to prevent severe hypoestrogenism leading to undesirable side-effects and loss of bone mineral density or development of endometrial hyperplasia resulting from continuous unopposed estrogen. A combination of GnRH agonists and testosterone or other androgens has also been investigated for male contraception for many years but without identification of a regimen, which results in sufficient suppression of spermatogenesis to provide effective contraception in all men safely and conveniently.5 An orally active nonpeptide GnRH antagonist, Elagolix, has been shown to have a dose-dependent effect on pituitary FSH/LH with a resultant dose related decline in serum estradiol levels.6 The ovarian response was variable within the women in the early clinical trial and expanded studies are warranted.
Collectively, clinical evaluation of GnRH analogues for contraception has been disappointing. Two major problems need to be resolved:
1. Partial suppression can lead to unopposed endogenous estrogen effect, resulting in endometrial hyperplasia and irregular bleeding.
2. Complete suppression, although effective for providing contraception and preventing endometrial complications, is associated with a hypoestrogenic state, which leads to unacceptable vasomotor symptoms, vaginal atrophy, and loss of bone mineral density.
To avoid these side-effects, a progestogen with or without estrogen has been added to GnRH therapy (add-back regimen) when these drugs are used for other gynecologic disorders (e.g. endometriosis, dysfunctional uterine bleeding).7However, the acceptability and long-term effects of such a regimen, for contraception, have not been sufficiently assessed. Additionally, GnRH analogues are costly and can be administered only intranasal or parenterally. The oral nonpeptide GnRH antagonist could circumvent some of these problems related to the use of GNRH analogues.
Steroid Hormones
Steroid hormones are known inhibitors of the hypothalamic–pituitary axis. Repeated cyclic administration of mestranol the 3-methyl ether of ethinyl estradiol has been shown to suppress both FSH and LH levels in the urine. Inhibition of pituitary gonadotropins is effected at the level of hypothalamus and pituitary. Ethinyl estradiol (50 μg/day) or mestranol (80 μg/day), given from day 5 through day 24 of the cycle, causes an abolition of the midcycle surge of serum FSH and LH. In sufficient doses, estrogen–progestogen combination oral contraceptives suppress plasma FSH and LH while eliminating the midcycle surge. The difference between follicular- and luteal-phase levels of FSH and LH is usually preserved.8
When administered together, estrogen–progestogen combination has many synergistic effects and can provide effective contraception with as little as 20 μg of ethinyl estradiol or less when combined with progestins.9, 10, 11 A significant issue with lower doses of ethinyl estradiol is the formation of ovarian cysts owing to less suppression of FSH. The true incidence and clinical impact of this finding is unknown.7
The suppressive effect of progestogens alone on FSH and LH release depends on the type and dosage of the steroid used. Norethindrone acetate (2.5 mg/day) given for 20 days beginning on cycle day 5 abolishes the midcycle peaks of both pituitary gonadotropins. Newer progestogens, such as desogestrel, gestodene, and norgestimate, have similar effects when administered in sufficient dosages. Medroxyprogesterone acetate (Depo-Provera 150 mg every 3 months) abolishes the midcycle surge of FSH and LH levels, although the tonic level of both gonadotropins remains within the normal range. With larger doses, suppression of tonic levels may also occur. Similarly, norethindrone enanthate (200 mg every 3 months) decreases FSH and LH to basal levels.
Oral administration of microdose progestogens or sustained release of these compounds in equivalent doses from the vaginal ring or various implants causes suppression of the midcycle peak of LH and FSH in some women. In other women, preovulatory FSH and LH surges may be completely abolished.8, 9, 10, 11 The basal levels of gonadotropins usually remain unchanged. The effect of oral progestins on ovulation in women is dose dependent and variable between studies.7, 12
In summary, estrogen–progestogen combination or progestogens alone administered orally, parenterally, vaginally, or transdermally affect the midcycle surge of LH and FSH without significantly altering baseline levels of these gonadotropic hormones. The net effect of these changes is the inhibition of ovulation to varying degrees, depending on the potency of the steroids and the dosage used. Recent advances in our understanding of the molecular mechanism of steroid hormone action provide new opportunities to improve the design of contraceptive pills. For many years, it was believed that only one gene was responsible for each type of sex steroid receptor and the same protein for estrogen, progestin, or androgen receptors was expressed in diverse tissues. Two different estrogen receptors (ERα and ERβ) have been identified that exhibit distinct functional differences within cells. Classical ER, now known as ERα, as well as ERβ both have binding domains that are critical to their ability to alter gene expression in the cell. The structure of these receptors is similar in these domains. The structure of the ligand-binding domain is also fairly well conserved between two receptors. However, the amino acid terminal regions of ERα and ERβ are different, and these regions contain areas that control the ability of the receptor to modulate gene expression. Thus, it appears that natural and synthetic steroids act in a tissue-specific manner, and their action may be determined by tissue-specific distribution of diverse accessory transcription factors co-activators and co-repressors.13, 14 These factors allow the manifestation of agonistic or antagonistic action of a given drug in a tissue-specific manner. Therefore, existing or new steroids with contraceptive potentials could have different agonistic or antagonistic actions in different tissues. Thus, it should be possible to design contraceptives capable of suppressing pituitary gonadotropin release without significant activity on breast, endometrium, or vascular tissues. For example, one might envision that women at high risk of having breast cancer could use designer contraceptives with preferential antiestrogen action on the mammary cells.14
Estrogen receptor agonist/antagonists also known as selective estrogen receptor modulators (SERMs) are synthetic estrogen-like substances that act as estrogen agonists in some tissues and as antagonists in others. Raloxifene and bazedoxifene, well known SERMs approved for use in postmenopausal women, have estrogen agonist activity on bone,15 and an estrogen antagonist activity on breast and endometrium.16 Raloxifene administered chronically in mice and rats increases serum LH levels, induces ovulatory arrest in rats resulting in anovulation and granulosal cell hyperplasia.17, 18 Bazedoxifene has similar effects in intact rodents and nonhuman primates.19 The preclinical studies indicating the anti-ovulatory potential of SERMs is interesting, but persistent elevation of LH in women could result in untoward adverse effects on the ovary.
Two types of progesterone receptors A and B have been identified. Progesterone antagonists and selective progesterone receptor modulators (SPRMs), which bind selectively to either A or B type, have been synthesized and have been clinically evaluated. Mifepristone, a progesterone antagonist, and ulipristal acetate, a SPRM, have been shown to have contraceptive potential.20, 21 Both compounds block the midcycle LH surge and have been used as emergency contraceptives (morning after pills).22 A major problem has been the progesterone receptor modulator induced changes in the endometrium which have retarded their clinical development.23
Other Peptides
Activin and inhibin are two peptides involved in modulating pituitary gonadotropin release and follicular development. Activin may have a potential for contraceptive development. Activin activity is modulated by follistatin another peptide that can inhibit or modify its activity on follicular development.24 Activin results in fewer antral follicles and subfertility in animal models.24 No clinical studies have been performed with these compounds.
Inhibin is a dimeric peptide composed of two dissimulator subunits: alpha and beta subunits. This peptide is produced in the granulosa cells of the ovary and suppresses FSH secretion. Its action is enhanced by insulin-like growth factor (IGF). Additionally, inhibin down regulates and reduces the number of GnRH receptors. The levels of inhibin vary reflecting follicular numbers. Although it modulates pituitary FSH activity, no data exist on its potential as a contraceptive.25
OVARY
Because the ovary is the site of gamete development and release in the female, it is an important target for contraceptive manipulation. Furthermore, the development and function of the corpus luteum are essential for the survival of the conceptus in the first trimester of pregnancy. Synthetic sex steroids may affect the ovaries indirectly by their inhibitory action on gonadotropin release or directly by altering the follicular environment. Suppression of midcycle surge of LH and FSH by estrogens, estrogen–progestogen combination, or progestogens alone has already been alluded to. The suppression of midcycle peaks of LH and FSH, however, does not lead invariably to anovulation. Approximately 40% of women using progestin only oral contraceptives ovulate.10, 12 Corpora lutea have been found in the majority (85%) of women receiving small daily doses of progestogens such as norgestrel, norethindrone, and quingestanol or megestrol acetate.8 These corpora lutea, however, are functionally abnormal, producing subnormal amounts of progesterone. Two mechanisms may be involved in the suppression of endogenous production of progesterone in the luteal phase in women treated with progestogens. First, the suppression of the gonadotropins during the follicular phase at midcycle may interfere with the normal stimulation and function of the corpus luteum. Second, progestogens may have a direct effect on steroidogenesis (luteolytic) in the corpora lutea.
Ovarian Follicle
The ovarian follicle plays a central role in reproductive function. The development of a healthy and normal-functioning ovarian follicle is in fact prerequisite to processes leading to ovulation and conception. The ovarian follicle is actively involved in the process of steroidogenesis and biosynthesis of a number of proteins and peptides, such as inhibin and activin.
Follicular fluid is also a rich source of many substances. It contains a low concentration of serum proteins less than 1,000,000 kd molecular weight, as well as many other substances, such as LH, FSH, estrogens, progestins, androgens, sex hormone binding protein, ovulating enzymes (plasminogens, proteases), and nonsteroidal ovarian factors. Follicular fluid is rich in steroid hormones, and the steroid concentration in follicular fluid greatly exceeds that in blood. Preovulatory follicles contain high levels of estrogens. Progesterone is found in fairly low levels in the nonovulating follicle, but its concentration rises in the late follicle. Follicles also contain measurable amounts of FSH, LH, and prolactin, always at concentrations lower than are found in the blood. FSH is found in all follicles that have started to form an antrum, whereas LH and progesterone appear together in preovulatory follicles. Prolactin and androgen levels fall with follicular maturation. Thus, follicles have their own hormonal environment, and it appears that the diffusion of steroids from the follicular fluid into blood is restricted, as evidenced by the very high estrogen levels maintained in the follicular fluid. Movement of steroids into follicular fluid from nearby follicles is also restricted, as can be seen by the low progesterone levels detected in follicles during the luteal phase, in the presence of an ipsilateral corpus luteum.26 Prostaglandins, which are involved in ovulation, are also found in follicular fluid of preovulatory follicles after LH surge.
In recent years, much research has been devoted to the identification and possible function of nonsteroidal ovarian factors. These substances play an important role in modulating intraovarian function and in fine-tuning gonadotropin action.26, 27, 28
Oocyte maturation inhibitor is a protein factor that can prevent oocyte maturation in vitro. Luteinization inhibition and stimulation factors are concerned with the process of luteinization. Various animal studies suggest that small follicles might contain a luteinization inhibition factor, whereas large follicles might contain a luteinization stimulation factor. These factors seem to be responsible for the development of LH receptors (or lack of them) and luteinization. Other putative intraovarian regulators may be involved in subtle in situ modulation of the growth of follicular structure and with intercompartmental communication, allowing for a tighter linking of different cellular populations. Potential intraovarian intercellular communication may take place by paracrine or autocrine action.28
The oocyte itself may control some factors leading to its maturation and ovulation. Two oocyte genes growth differentiation factor-9 and bone morphogenetic protein-15 have been found to be critical for ovulation in sheep.29 The fatty acid elongase 1 gene was one of 43 identified in rodents as having a true ovulation/selective gene expression pattern.30 To dat,e no clinical utility of these genes as contraceptives has been reported.
Among putative intraovarian regulators, several have received considerable attention and may conceivably be a target for fertility regulation.26, 27, 28 Insulin-like growth factor-I (IGF-I) is a polypeptide involved in amplification of gonadotropin hormonal action. Recent information indicates that IGF-I, which is produced by granulosa cells, binds to the interstitial cells that are not a site of IGF-I gene expression but are endowed with receptors. Thus, it appears that IGF-I may engage in intracompartmental communication in the interest of coordinated follicular development.31
Another polypeptide, transforming growth factor-X, has proved to be a potent inhibitor of gonadotropin-supported granulosa cell differentiation.
Transforming growth factor-3T is yet another polypeptide that is produced by both granulosa and theca interstitial cells and has been shown to alter profoundly the proliferation and differentiation of rat granulosa cells. At this time, however, the potential significance of transforming growth factor β1 to human ovarian physiology remains unknown. Mention should also be made of basic fibroblast growth factor, interleukin-1, and tumor necrosis factor-2, which are thought to be involved in granulosa cell development and regulation of luteal cells.27, 28
Contraceptive Implications
The dominant preovulatory follicle has a precise hormonal milieu. It is conceivable that a disturbance of this environment might lead to follicular atresia or anovulation. Both combination oral contraceptives and progestogen-only formulations might well bring about an alteration of the follicular environment in addition to their other effects.
The specificity of the factors discussed and their presumed localization of action suggest a number of new targets for contraception.27 For example, if the LH surge were prevented from inducing withdrawal of oocyte maturation inhibitor from the oocyte in the dominant follicle, all of the endocrine events of the cycle would occur, but the oocyte would not be viable. Injection of oocyte maturation inhibitor to reach the oocyte during the time when the surge is occurring could prevent resumption of meiosis.
Follistatin injected into several animal species has been shown to prevent FSH secretion and follicular growth. Similarly, inadequate formation or maintenance of corpora lutea might result from local overproduction of luteinization inhibition factor. Currently, the factors influencing the control of synthesis and release of follicular peptide are not well understood, but considerable efforts are being made to obtain such information.
The ovarian GnRH receptors may be considered an excellent potential target for contraception. GnRH and its agonists may affect the ovarian function in two different ways:
1. Chronic stimulation of pituitary gonadotropes by potent GnRH agonists may cause cellular desensitization, with a subsequent decrease in circulating gonadotropins.
2. GnRH may directly suppress ovarian steroidogenesis, ovulation, and corpus luteum function.
Similarly, the GnRH antagonists can block pituitary gonadotropin secretion; thus, timed administration of GnRH agonists or antagonists can block ovulation, follicular development, or corpus luteum function, depending on the phase of the cycle when the drug is administered.
Oocytes undergo meiosis I during follicular maturation and then following the LH surge the oocyte undergoes meiosis II immediately prior to ovulation. The resumption of meiosis prior to ovulation requires a reduction in cyclic AMP (cAMP) that is the result of the enzyme phosphodiesterase 3A (PDE3). Blocking or inhibiting PDE3 has been found to inhibit meiosis in both human and monkey oocytes.32 A pilot study using ORG 9935 a PDE 3A inhibitor in naturally mating monkeys failed to show efficacy as a contraceptive compared to the placebo group.32 A possible explanation was that the serum levels of RG 9935 were lower in the animals that become pregnant. Further investigation of this approach for contraception is needed.
Interference with Corpus Luteum Function
Corpus luteum function is essential for implantation of the conceptus and its further development in the first 8 weeks of gestation. Thereafter, the hormonal burden for the maintenance of pregnancy is shifted to the placenta. The corpus luteum is, therefore, an attractive target for fertility regulation. Disruption of luteal function may prevent implantation. After implantation, induction of luteolysis is likely to cause an arrest of fetal development and abortion. Studies in experimental animals and in humans have shown that GnRH analogues may exert luteolytic activity. For example, administration of GnRH agonists to Rhesus monkeys 3–5 days postovulation has been found to cause a significant shortening of the luteal phase and decrease in serum progesterone values. Administration of human chorionic gonadotropins (hCGs) prevented the shortening of the luteal phase in animals treated with the analogue. In rats, administration of GnRH antagonists interferes with pregnancy. In monkeys, however, initial attempts to alter early pregnancy with GnRH antagonists have not been successful. In humans as well, GnRH analogues induce luteolysis when given 5–8 days after the LH peak. The early corpus luteum seems to be refractory to luteolytic activity of the agonists. However, the luteolysis induced by GnRH-agonist treatment is prevented by exogenous hCG. In addition, simultaneous administration of GnRH analogues and hCG does not cause luteolysis. These studies suggest that rising levels of hCG in early pregnancy may oppose the GnRH induced luteolysis.
Interference with Follicular Rupture
The precise sequence of events leading to follicular rupture and extrusion of oocytes has not been clarified. In several animal species, including subhuman primates, prostaglandin F2α and prostaglandin E2 may play an important role. Prostaglandin synthetase inhibitors, such as indomethacin and ibuprofen, have been shown to prevent follicular rupture and ovulation after hCG administration, while the ovaries continue to undergo luteinization. Furthermore, prostaglandins have luteolytic activity, so their administration in the early luteal phase may interfere with the function of corpus luteum and prevent survival of the conceptus.
Prostaglandins and Cyclooxygenase
Cyclooxygenase (COX) enzymes are important in prostaglandin biosynthesis and prostaglandins play a significant role in primate ovulation.33, 34 Nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit COX enzymes and ovulation in animal species.35, 36 Cox-2 can be selectively inhibited by COX-2 specific NSAIDs. Use of meloxicam, a semi-selective COX-2 inhibitor, delays ovulation and has potential as an emergency contraceptive method.37, 38, 39 However, the continued administration of Celebrex, a specific COX-2 inhibitor, has failed to show significant inhibition of ovulation in normal women.40 This area deserves further investigation relative to the role and efficacy of prostaglandins in ovulation.
Another potential intraovarian factor target for control of follicular rupture is RAS. RAS involvement in oocyte maturation and ovum release has been suggested by several studies. High levels of renin and angiotensin are found in preovulatory follicular fluid, and these substances are believed to be involved in the maturation of the oocyte and in ovulation, either directly or through other ovarian regulators. It has also been suggested that angiotensin II may play a role in the formation of the corpus luteum and steroid secretion by luteal cells.27, 41 In fact, the administration of saralasin acetate, an RAS antagonist, in animal studies has been shown to block the reproductive process. Clinical studies are required to determine the feasibility of such an approach in a human model.
Vascular endothelial growth factor (VEGF) and angiopoietin are involved in angiogenesis. Angiogenesis is crucial for follicular growth and corpus luteum function. Antagonists to these two factors when injected into the preovulatory follicle of primates suppressed ovulation and luteinization in monkeys.42
Hypoxia inducible factor genes appear to play a role in ovulation. Ovulation in rodents is inhibited by echinomycin, a small molecule that blocks the activity of the hypoxia inducing factors on metalloproteinases, and resulted in a reduced VEGF A, the latter involved in angiogenesis.43 Although of interest this pathway requires further investigation.
FALLOPIAN TUBES
Oviducts have several major functions in human reproduction: transporting spermatozoa to the site of fertilization, picking up the ovum, and transporting the conceptus to the uterus. Additionally, the fallopian tubes are the sites of fertilization and cleavage, and tubal secretion may be important for nutrition and development of the embryo. Interference with any of these functions might provide contraception.
Surgical Interruption of Fallopian Tubes
Surgical interruption of the tubes is one of the most widely used techniques of permanent sterilization. A variety of surgical techniques have been devised to interrupt the continuity of the fallopian tubes. The majority of these methods aim at removing a segment of the tube and ligating the ends. Fallopian tubes may also be ligated or fulgurated endoscopically. With laparoscopy, the tubes may be ligated (Fallop ring, clip) or fulgurated. The use of bipolar electrodes has improved the safety of thermocoagulation considerably. Finally, the tubes may be occluded hysteroscopically using electrocoagulation, thermocoagulation, mechanical devices, cryosurgery, or laser. The success of surgical interruption of the tubes varies considerably depending on the type of procedure, the population treated, and the expertise of the operator.44
A new development is the insertion of devices via hysteroscopy into the uterine tubal ostia. Essure and Adiana are both devices that initiate a local inflammatory process and result in sclerosis of the tubal lumen.44 A drawback to their use in large populations is the need to insert the devices under direct visualization via the cervix and to document tubal obstruction 3 months after insertion with a hysterosalpingogram.
Medical Approaches to Tubal Occlusion
Delivery of pharmacologically active agents or sclerosing substances to occlude the fallopian tube has many attractive features. These substances may be delivered to the tubal ostia and uterotubal junction using only local anesthesia with hysteroscopy or blindly on an outpatient basis with the possibility of use by trained paramedic personnel.45 During the past two decades, several interesting systems have been developed using a variety of chemical agents. Among these, only silver nitrate, quinacrine, silicone rubber, phenol, and methylcyanoacrylate have reached the stage of significant clinical trials or are being used in developing countries.46, 47
Silver nitrate, 10%, 15%, and 20%, has been used clinically either as an ointment or as a derma-based carrier transcervically. These trials have been associated with a considerable degree of complications and side-effects. Silver acetate has also been delivered in an alginate system as a sclerosing agent. Several groups have attempted transcervical delivery of silicone rubber.45
In China, a relatively large number of women have been treated with several formulations of liquid phenol and mucilage. These compounds were administered by inserting a cannula transcervically up to the tubal ostia blindly on an outpatient basis. The treated women apparently experienced only mild side-effects. Quinacrine has also been studied extensively as a tube-blocking agent. An interesting substance that has been evaluated is methylcyanoacrylate. Richart and others developed an ingenious delivery system (Femcept) to deliver methylcyanoacrylate to the uterotubal junction with interesting preliminary results.46
Pilot experiments in rodents and monkeys with polidocanol a sclerosing agent have found disparate outcomes. The rodents demonstrated inflammation and changes in the reproductive tract while no physical changes were found in the monkey after intrauterine infusion of a 1% solution of polidocanol.48 The lack of effect in the monkey was thought to be due to several problems including the concentration used, duration of application, and need for repeated treatments.48
A product termed reversible inhibition of sperm under guidance (RISUG) is styrene maleic anhydride–dimethyl sulphoxide which contains iron and copper, and could be a reversible contraceptive in either men or women based on pr clinical animal studies.49
In summary, a number of effective systems for the delivery of pharmacologically active agents to the fallopian tubes, either blindly or under direct vision, have been developed. Unfortunately, all are in an experimental stage, and none have undergone extensive clinical trials. The current need is to integrate the delivery system with a safe, effective agent that consistently produces tubal closure.
Tubal Fluid
Within the oviducts, the oocyte is suspended in fluid formed by secretion of the tubal epithelium, which is also the medium for capacitation of sperm, fertilization, cleavage, and maturation of the morula. The tubal fluid, composed of serum transudate and specific secretion containing some unique proteins, is regulated quantitatively and qualitatively by ovarian sex steroids.
The tubal mucosa is made of ciliated and nonciliated secretory cells. Nonciliated cells are responsible for tubal secretion, whereas ciliated cells are believed to be involved in gamete transport. The function of these cells is also regulated by estrogen and progesterone.
Finally, tubal contractions are known to be important in facilitating the mixing of tubal contents, helping to denude the ovum, promoting fertilization by increasing egg–sperm contact, and regulating egg transport.
Estrogen and progesterone affect the morphology and function of the tubal epithelium, tubal secretion, and tubal contraction. Thus, steroidal contraceptives also exert their effect on the function and internal milieu of the tube. The effect of these steroids varies with their relative proportions and the timing of their administration.50
Nonsteroidal agents, such as gonadotropins, prostaglandins, and other drugs influencing the contraction of smooth muscles (e.g. reserpine); can also alter egg transport and the action of the ampullary, isthmic and uterotubal junctions. These compounds may accelerate or impede the rate of egg transport. Could inhibition or modification of tubal cilia beat and/or tubal motility result in an effective contraceptive is an open question.51, 52
UTERUS
The uterus has three major functions in human reproduction: sperm transport, implantation, and maintenance of gestation. All of these activities may potentially be altered to produce contraception. Steroidal contraceptives affect both myometrial and endometrial histology and alter the functional activity of the uterus.
The nonpregnant uterus exhibits constant spontaneous contractile activity, which varies during different phases of the menstrual cycle and is regulated by ovarian hormones. Uterine contractions seem to have an important function in sperm transport as well as implantation of the blastocyst.53 The pattern of myometrial activity can be altered by the administration of estrogen–progestogen contraceptives. Similarly, prostaglandins are known to affect uterine contractions. The potential use of other pharmacologically active agents that may affect the myometrium, as a target organ has not been explored.
Ovarian hormones bring about well-recognized histologic changes in the endometrium during the menstrual cycle. These morphologic changes are associated with complex biochemical and enzymatic alterations that may play an important role in the process of implantation.
The effect of sex steroids on the endometrium has been investigated extensively and depends on the type and dosage, as well as the duration, of administration. The effect of combination preparations consists in rapid progression from proliferation to early secretory changes within a few days after administration of the compounds. By midcycle, a varying degree of mixed hormonal effect is observed. Thereafter, the endometrium shows regressive changes characterized by a relatively compact stroma dotted with sparse atrophic glands covered by a cuboidal or flattened epithelium. With prolonged use, the endometrium becomes progressively thin and inactive in most women.8, 9 The response of endometrium to different types and doses of pure progestogens given orally or administered through various implants is variable, ranging from proliferative and inactive endometrium to secretory and irregular (mixed) secretory changes.8 Histochemical studies have shown marked alterations of various endometrial enzymes in women receiving steroidal contraceptives. These changes are interpreted to indicate that the endometrium is an important site of contraceptive action of sex steroids, which inhibit implantation even when administered at dosages that do not inhibit ovulation. In addition to their cyclic administration, estrogen–progestin combination pills or progestin-only formulations have been used for emergency contraception. The usual regimen consists of the ingestion of pills containing 0.1 mg ethinyl estradiol and 1.0 mg norgestrel in two doses 12 hours apart starting within 72 hours of unexpected sexual intercourse. The progestin-only method consists of the ingestion of 0.75 mg levonorgestrel in two doses taken in the same manner. Therefore, these preparations most likely act in a manner similar to cyclic administration of these steroids by inhibiting or delaying ovulation or inducing deficient corpus luteum function, depending on the day of the cycle that they are administered, histologic and biochemical changes within the endometrium resulting in failure of nidation, and interfering with tubal transfer of sperm, egg, or embryo. Up to 98% of women menstruate by 21 days after treatment.54, 55
Another approach to alter endometrial receptivity is the use of antiprogestin compounds. These 19 nor-steroids occupy progesterone receptor sites without acting as an antagonist and prevent the natural hormone from binding to these sites and exerting its normal function (i.e. endometrial secretory changes in preparation for implantation or maintenance of gestation or both).54 Other biologic activities of antiprogestins include weakening of gestational sac attachment to decidua, increased prostaglandin accumulation, and sensitization of the uterus and the cervix to their effect.
The first of these progesterone antagonists, RU486 (mifepristone), was synthesized in 1980. The drug has been used extensively and successfully in several countries. Mifepristone has a high affinity for progesterone receptors, but is not a pure antagonist. In fact, in the absence of progesterone, it can act as a partial agonist. Mifepristone is used primarily to induce abortion in early pregnancy. The combination of mifepristone and prostaglandin given 48 hours later has resulted in a rate of complete abortion approaching 90–100% in pregnancies of fewer than 50 days.54, 55
Antiprogesterone compounds may also be used for contraception. Clinical studies indicate that their administration during the preovulatory phase inhibits LH surge and induces menses in the late luteal phase, regardless of whether fertilization has occurred. Finally, mifepristone has been used as a postcoital contraception when given within 72 hours after unprotected intercourse.54, 55, 56 Effectiveness of a continuous low dose administration of mifepristone orally as a contraceptive has been reported,57 but its utility is limited due to other nonmedical concerns.
Intrauterine devices (IUDs) are now used extensively for fertility control. The contraceptive effectiveness of IUDs is believed to be related to the production of a local sterile inflammatory reaction within the uterine cavity. The extent of this inflammatory process is directly related to the surface area of the IUD. The sterile inflammatory reaction appears to inhibit sperm transport through the uterus and tubes, and its ability to fertilize an ovum.10, 58
In addition to their action as a foreign body within the uterine cavity, IUDs may also be used for local delivery of steroids, spermicidal agents, or other anti-fertility or pharmacologically active substances. These substances (e.g. copper, progesterone) may be used to improve contraceptive efficacy or to diminish side-effects and complications associated with IUD use.59, 60 The addition of levonorgestrel to the IUD frame has resulted in an effective contraceptive with an added benefit of diminishing endometrial bleeding. Lower doses of the levonorgestrel releasing intrauterine system are now available releasing 14 µg per day with efficacy for 3 years.61
CERVIX
Because of its important function in sperm transport, the uterine cervix is considered a major site of action for some existing contraceptives and presents several possibilities for the development of new fertility-controlling methods.
Sex Steroids
The secretory activity of cervical epithelium is controlled by sex hormones. An increase in the amount of endogenous estrogen during the preovulatory phase of the cycle or the administration of synthetic estrogens produces copious amounts of thin, watery, alkaline, acellular cervical secretion with intense ferning, spinnbarkeit, and sperm receptivity.
Endogenous progesterone during the luteal phase of the cycle or in pregnancy produces scanty, viscous, cellular mucus with low spinnbarkeit and no ferning. Spermatozoa are unable to penetrate progestational cervical mucus. Other constituents of cervical mucus, such as proteins, enzymes, and electrolytes, are sensitive to hormonal changes. The surface tension and conductivity of mucus are also controlled by estrogen and progesterone. Administration of estrogen to postmenopausal or castrated women and to those with supracervical hysterectomy and excised ovaries causes a significant increase in the amount and translucency of the mucus. Administration of progesterone alone has no effect. Almost all synthetic oral and parenteral progestogens, alone or in combination with estrogen, to some degree, inhibit mucorrhea and sperm penetration through the cervical mucus. Under the influence of these compounds, cervical mucus becomes highly viscous and cellular and is secreted in scant quantities. The ferning pattern disappears, and spinnbarkeit is markedly reduced. The normal preovulatory decrease in albumin, enzymes, and sialic acid and the increase in mucins are altered. The pH, however, is not significantly changed; this property of progestogens is of practical importance. The contraceptive effectiveness of progestogen-only contraceptives administered orally or by implants (e.g. levonorgestrel, Norplant system) depends largely on their ability to produce cervical mucus hostile to sperm penetration.8, 9, 10
Local Administration of Progestogens and Enzyme Inhibitors
Progestogens are known to diffuse out of silastic devices and to be absorbed by surface epithelium of the reproductive tract. An intracervical progestogen-releasing device has been developed and is used on a limited basis.60, 62, 63 The device releases approximately 10 μg levonorgestrel per day.60 The continuation rate, incidence of unintended pregnancies, and incidence of removal due to pain or bleeding are comparable to those of the CU-T-200 IUD.60, 62, 63
Several enzymes in the acrosomal cap of spermatozoa have been identified as essential to the process of fertilization. Among these, acrosin appears to be involved in penetration of sperm in the zona pellucida. The development of acrosin inhibitors in the form of vaginal or cervical contraceptives has been considered but has not yet been successful.
Administration of Pharmacologically Active Agents
Certain organic and inorganic substances diffuse readily through the cervical epithelia and appear in cervical secretion. Notable among these are sex steroids, serum-type proteins, steroids, electrolytes, iodine, thyroxine, quinine, and others. With the exception of sex steroids, however, systemic use of these agents to affect cervical contraception has not been promising.
Ovulation Prediction and the Periodic Abstinence Method of Contraception
Changes in cervical mucus during the menstrual cycle have been used to determine the time of ovulation for the purpose of the sexual abstinence method of family planning. The cervical mucus method relies on self-observation and perception of midcycle mucorrhea for ovulation timing. Five phases of the cervical mucus pattern are recognized: phase 1, dry days; phase 2, early preovulatory days; phase 3, wet days (maximum preovulatory mucus secretion); phase 4, postovulatory days; and phase 5, late postovulatory days. The fertile or unsafe period is presumed to start on the first day in which postmenstrual mucus is observed (phase 2) and to continue until the fourth day after the clear lubricative cervical mucus (peak day) appears, a period that can last for 7–14 days. All subsequent days are considered infertile and safe for sexual intercourse.
Billings and associates reported a high degree of effectiveness in well-motivated couples who had been adequately trained.64 In a randomized, prospective study performed in the Los Angeles area during the year after formal entry into the study, 36.6% of users withdrew from the program and 24.8% became pregnant.65 In another multicenter clinical trial conducted by the World Health Organization in five countries, a pregnancy rate of 22.5 per 100 women per year was observed among those who learned the method.66 Interestingly, method failure yielded only a 3.1% pregnancy rate, but incorrect use of the method resulted in a pregnancy rate of 86.4%. Thus, proper and consistent use of this method is highly effective, but it requires considerable discipline by the couple.
To improve on the objectivity of the cervical mucus method and to narrow down the number of days when abstinence needs to be practiced, attempts have been made to use one of the constituents of cervical mucus, such as an enzyme (alkaline phosphatase, guaiacol peroxidase), as a marker to be detected by the patient using a simple colorimetric or dip-stick method. Preliminary experiments along these lines, however, have not met with overwhelming success.
Cervical Cap
Occlusive devices to prevent sperm migration through the cervix have been used for many years but have emerged as a safe and practical alternative to vaginal diaphragm. The cervical cap has several advantages over the diaphragm. It can be left in place for up to 48 hours and it need not be used with a spermicide. However, a tablespoonful of spermicide placed in the cap before application is reported to increase its efficiency (to a 6% failure rate in the first year and to prolong wearing time).
VAGINA
Approximately 50–500 million sperm are deposited in the vagina during normal coitus. Human semen coagulates immediately after ejaculation and traps most spermatozoa until seminal proteolytic enzyme brings about liquefaction and allows the sperm to penetrate the cervical mucus on their way to the upper reproductive tract. Because the vagina is an accessible site, it provides an excellent target for barrier contraceptives and spermicides to destroy spermatozoa and prevent sperm penetration in cervical mucus. Traditionally, diaphragms of various designs along with spermicidal jellies have been used for this purpose. Recent attempts to improve on current barrier female contraceptives have resulted in approval of the female condom, FemCap, Lea’s shield, and the development of SILCS diaphragm.67, 68, 69, 70
Spermicides may be used alone as creams, foams, and suppositories. More recently, attention has been focused on the development of devices or agents that may play the dual function of preventing sexually transmitted diseases and providing contraception. The female condom is an example of such an approach.
The vaginal mucosa has the ability to allow many pharmacologically active substances, including steroids, to cross through it and to reach the systemic circulation. Therefore, the administration of sex hormones by this route may produce contraception similar to their oral, transdermal, or parenteral administration. The estrogen–progestogen-containing vaginal ring is an example of this approach. The NuvaRing® vaginal ring is a flexible, colorless device releasing on average 120 μg of etonorgestrel and 15 μg of ethinyl estradiol per day. The ring has a functional life of 3 weeks. It is used for 3 weeks continuously followed by a 1-week ring-free period. A new ring is used for each cycle. Administration of combination estrogen–progestogen by the vaginal ring inhibits ovulation much the same way as the oral preparations. Additionally, there may be alterations of cervical mucus and endometrial changes contributing to contraceptive efficacy.71 The use of a vaginal ring for contraceptive and prevention of HIV transmission is a goal of the CONRAD program entitled Dual prevention technology http://www.conrad.org/prevention.html.72, 73 More information is required about the physiology of the vagina and ways to develop innovative methods of fertility control at this site.
IMMUNOLOGIC FERTILITY CONTROL
Immunologic inhibition of reproductive function is an attractive approach to fertility regulation because it can be applied by paramedic personnel to relatively large-population groups.
Numerous proteins of the reproductive system have been shown to be immunogenic, and immune response to many of them has been reported to disrupt fertility. The development of a safe and effective vaccine that can be mass produced is not a simple task. Some potential problems include: (1) identification of a unique and specific antigen for the development of antibody; (2) development of a vaccine that results in an infertile state for a reasonable length of time (at least 1 year and no longer than 3 years); (3) potential reversibility; and (4) cross-reactivity of antibodies with other tissue antigens.
Ideally, the antigens to be inhibited by vaccination should be present in the recipient only intermittently and in relatively low quantities. Examples of such antigens in the female are sperm antigens after coitus and placenta-specific antigens during early pregnancy.
The reproductive epithelium in the female is capable of responding to various antigenic stimuli. Biosynthesis of immunoglobulin G (IgG) and immunoglobulin A (IgA) in the uterine, cervical, and vaginal tissues of the rabbit and in the cervical tissues of women has been reported. These antibodies may remain tissue bound or may be secreted in the reproductive tract fluids, where their concentration is usually many times less than that of serum. Early experiments involving immunization of female animals with sperm or testicular components established the potential usefulness of sperm antigens for immunocontraception.74, 75, 76, 77, 78, 79
Several antigenic systems to mammalian (including human) semen and spermatozoa are known to be present. These include species-specific and sperm-specific antigens, transplantation antigens, sperm-coating antigens, and seminal plasma antigens. These antigens elicit various responses in the female reproductive tract. Numerous studies have been conducted in laboratory animals showing inhibition of fertility from sperm immunizations.80 Purified preparations of at least three sperm enzymes have been evaluated as potential antigens for fertility control. Two acrosomal enzymes, hyaluronidase and acrosin (trypsin-like enzyme), have been shown to be immunogenic after immunization. Unfortunately, the fertility rates of the actively immunized animals were not significantly affected by this treatment. A third sperm enzyme, LDH-C4 (LDH-X), has shown more promise in preliminary experiments.81 Other relevant sperm antigens with contraceptive potentials have also been identified.82 The failure of contraceptive vaccines results from the inability of antibodies to a single antigen, to completely inhibit fertilization, because several antigens may act cooperatively, and the inability of systemic immunization to deliver an optimal immune response to the reproductive tract. It appears that a number of sperm molecules are involved in sperm/egg interaction and their cooperation determines the overall success or failure of fertilization. However, this does not mean that the removal or blockage of a single essential component cannot inhibit the entire system.82 The zona pellucida, an acellular, gelatinous-like layer that surrounds the egg, is attracting increased attention as a target for regulating fertility. Major functions of the zona pellucida in fertilization include sperm recognition (species-specific) and prevention of polyspermia. Species-specific sperm receptor sites located on the surface of the zona are responsible for preventing sperm of one species from fertilizing eggs of another species. Homologous and heterologus antibodies to the zona pellucida have been produced experimentally in several species including subhuman primates to achieve immunocontraception. These antibodies have been shown to block fertilization in vitro and in vivo and to prevent implantation. Unfortunately ovarian function is often disturbed in immunized animals.83, 84, 85 Extension of these studies to humans may open a new era in contraceptive technology.
Over the past few years, the application of molecular biologic techniques to the zona immunocontraceptive area has resulted in rapid accumulation of significant new information on zona composition and its amino acid sequence. These recent findings have shown that although zona antigens are tissue-specific, they are not species-specific and that antibodies developed against the zona of one species crossreact in varying degrees with the zona of other species.86
An approach currently pursued to identify and delineate zona epitopes for contraceptive vaccine development consists of immunization using synthetic zona peptide. Using this approach, a site-directed antipeptide serum developed against an amino acid synthetic peptide corresponding to an amino terminal sequence of porcine ZP3X has been reported to interfere with in vitro spermatozoa zona attachment.79 The placental protein hormones have also been used for immunization. These hormones resemble those produced by the pituitary gland, and antibodies to placental hormones may thus crossreact with their respective pituitary hormones.87
Some protein hormones have an α- and β-subunit. The β-subunit of hCG differs from that of LH, which has the same biologic activity. Antibodies against the β-subunit of hCGs have been developed in several animal species and in women, with resulting inhibition of implantation.87 The menstrual cycle is not usually altered, indicating that the functions of the pituitary gonadotropins have not been suppressed. Immunization against hCG has now been extended to clinical trials for the suppression of human fertility. Pure preparations of hCGβ have been obtained for immunization, and the immunogenicity of hCGβ has been increased by chemical modification or by coupling, as a hapten to a larger molecule.86 Immunization against hCGβ may involve some potential problems, such as the effect on T- and B-cells, lack of efficacy in multiparous females previously exposed to endogenous hCG, and development of antibodies with low affinity leading to insufficient protection by anti-hCGβ. It is clear that these problems would have to be resolved before large-scale human studies could be undertaken and a current review suggests that we are close to having a human product.88 Several other antigenic candidates for vaccine development have been identified, and their potential use is being investigated.87
CONCLUSION
Female contraception offers several intriguing targets that need to be explored. The development of a nonsteroidal contraception is being vigorously pursued in several laboratories, but as yet is still in the preclinical phase.
REFERENCES
Hotchkiss J, Knobil E: The menstrual cycle and its neuroendocrine control. In Knobil E, Neill JD (eds): The Physiology of Reproduction, pp 711–749, Vol 2, 2nd ed, Chap 48. New York, Raven Press, 1994 |
|
Moghissi KS: Gonadotropin releasing hormones: Clinical applications in gynecology. J Reprod Med 35: 1097, 1990 |
|
Schmidt-Gollwitzer M, Hardt W, Schmidt-Gollwitzer K et al: The contraceptive use of Buserlin, a potent LHRH agonist: Clinical and hormonal findings. In Zatuchni GI, Shelton JD, Sciarra JJ (eds): LHRH peptides as female and male contraceptives, pp199–215. Philadelphia, Harper & Row, 1981 |
|
Berquist C, Nillius SJ, Wide L: Inhibition of ovulation in women by intranasal treatment with luteinizing hormone releasing hormone agonist. Contraception 19: 497, 1979 |
|
Anderson RA: Hormonal contraception in the male. Br Med Bull 56: 717, 2000 |
|
Struthers RS, Nicholls AJ, Grundy J, et al. Suppression of gonadotropins and estradiol in premenopausal women by oral administration of the nonpeptide gonadotropin-releasing hormone antagonist elagolix. Journal of clinical endocrinology and metabolism 2009;94:545-51. |
|
Moghissi KS, Schlaff WD, Olive DL, Skinner MA, Yin H. Goserelin acetate (Zoladex) with or without hormone replacement therapy for the treatment of endometriosis. Fertility and sterility 1998;69:1056-62. |
|
Moghissi KS. Effects of steroidal contraceptives on the reproductove system. In: Hasfez E, ed. Human Reproduction, Conception and Contraception. 2nd ed. Hagerstown: Harper & Row; 1980:529. |
|
Moghissi KS. Microdose progestogens for contraception. Regulation of Human Fertility 1976;57. |
|
Rivera R, Yacobson I, Grimes D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. American journal of obstetrics and gynecology 1999;181:1263-9. |
|
Killick S, Eyong E, Elstein M. Ovarian follicular development in oral contraceptive cycles. Fertility and sterility 1987;48:409-13. |
|
Rice CF, Killick SR, Dieben T, Coelingh Bennink H. A comparison of the inhibition of ovulation achieved by desogestrel 75 micrograms and levonorgestrel 30 micrograms daily. Human reproduction 1999;14:982-5. |
|
Hall JM, McDonnell DP. Coregulators in nuclear estrogen receptor action: from concept to therapeutic targeting. Molecular interventions 2005;5:343-57. |
|
Hsueh AJ. Designer contraceptive pills. Human reproduction 1995;10:1997-2000. |
|
Hadji P. The evolution of selective estrogen receptor modulators in osteoporosis therapy. Climacteric : the journal of the International Menopause Society 2012;15:513-23. |
|
Gambacciani M. Selective estrogen modulators in menopause. Minerva ginecologica 2013;65:621-30. |
|
Long GG, Cohen IR, Gries CL, Young JK, Francis PC, Capen CC. Proliferative lesions of ovarian granulosa cells and reversible hormonal changes induced in rats by a selective estrogen receptor modulator. Toxicologic pathology 2001;29:403-10. |
|
Banks PK, Meyer K, Brodie AM. Regulation of ovarian steroid biosynthesis by estrogen during proestrus in the rat. Endocrinology 1991;129:1295-304. |
|
Wright DJ, Earnhardt JN, Perry R, et al. Carcinogenicity and hormone studies with the tissue-selective estrogen receptor modulator bazadoxifene. Journal of cellular physiology 2013;228:724-33. |
|
Baird DT, Brown A, Cheng L, et al. Mifepristone: a novel estrogen-free daily contraceptive pill. Steroids 2003;68:1099-105. |
|
Chabbert-Buffet N, Pintiaux A, Bouchard P. The immninent dawn of SPRMs in obstetrics and gynecology. Molecular and cellular endocrinology 2012;358:232-43. |
|
Gemzell-Danielsson K, Rabe T, Cheng L. Emergency contraception. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology 2013;29 Suppl 1:1-14. |
|
Mutter GL, Bergeron C, Deligdisch L, et al. The spectrum of endometrial pathology induced by progesterone receptor modulators. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2008;21:591-8. |
|
Xia Y, Schneyer AL. The biology of activin: recent advances in structure, regulation and function. The Journal of endocrinology 2009;202:1-12. |
|
Tsigkou A, Luisi S, Reis FM, Petraglia F. Inhibins as diagnostic markers in human reproduction. Advances in clinical chemistry 2008;45:1-29. |
|
Adashi EY. Intraovarian peptides. Stimulators and inhibitors of follicular growth and differentiation. Endocrinology and metabolism clinics of North America 1992;21:1-17. |
|
Schwartz NB. Novel peptides in ovarian follicular fluid: implications for contraceptive development. Research frontiers in fertility regulation : RFFR / PARFR 1982;2:1-11. |
|
J Yeh EA, Z Rosenwaks The ovarian follicle: Life cycle of a pelvic clock. In: J Yeh EA, Z Rosenwaks ed. Reproductive Endocrinology, Surgery and Technology. Philadelphia: Lippincott-Raven; 1996:212-34. |
|
McNatty KP, Smith P, Moore LG, et al. Oocyte-expressed genes affecting ovulation rate. Molecular and cellular endocrinology 2005;234:57-66. |
|
Hourvitz A, Gershon E, Hennebold JD, et al. Ovulation-selective genes: the generation and characterization of an ovulatory-selective cDNA library. The Journal of endocrinology 2006;188:531-48. |
|
Adashi EY. The IGF family and folliculogenesis. Journal of reproductive immunology 1998;39:13-9. |
|
Jensen JT, Stouffer RL, Stanley JE, Zelinski MB. Evaluation of the phosphodiesterase 3 inhibitor ORG 9935 as a contraceptive in female macaques: initial trials. Contraception 2010;81:165-71. |
|
Duffy DM, Dozier BL, Seachord CL. Prostaglandin dehydrogenase and prostaglandin levels in periovulatory follicles: implications for control of primate ovulation by prostaglandin E2. The Journal of clinical endocrinology and metabolism 2005;90:1021-7. |
|
Hester KE, Harper MJ, Duffy DM. Oral administration of the cyclooxygenase-2 (COX-2) inhibitor meloxicam blocks ovulation in non-human primates when administered to simulate emergency contraception. Human reproduction 2010;25:360-7. |
|
Salhab AS, Gharaibeh MN, Shomaf MS, Amro BI. Meloxicam inhibits rabbit ovulation. Contraception 2001;63:329-33. |
|
Gaytan M, Morales C, Bellido C, Sanchez-Criado JE, Gaytan F. Non-steroidal anti-inflammatory drugs (NSAIDs) and ovulation: lessons from morphology. Histology and histopathology 2006;21:541-56. |
|
Bata MS, Al-Ramahi M, Salhab AS, Gharaibeh MN, Schwartz J. Delay of ovulation by meloxicam in healthy cycling volunteers: A placebo-controlled, double-blind, crossover study. Journal of clinical pharmacology 2006;46:925-32. |
|
Jesam C, Salvatierra AM, Schwartz JL, Croxatto HB. Suppression of follicular rupture with meloxicam, a cyclooxygenase-2 inhibitor: potential for emergency contraception. Human reproduction 2010;25:368-73. |
|
McCann NC, Lynch TJ, Kim SO, Duffy DM. The COX-2 inhibitor meloxicam prevents pregnancy when administered as an emergency contraceptive to nonhuman primates. Contraception 2013;88:744-8. |
|
Edelman AB, Jensen JT, Doom C, Hennebold JD. Impact of the prostaglandin synthase-2 inhibitor celecoxib on ovulation and luteal events in women. Contraception 2013;87:352-7. |
|
Adashi EY, Rock John A, Rosenwaks Zev. The ovarian follicle: Life cycleof a pelvic clock. In: Adashi EY, Rock John A, Rosenwaks Zev, ed. Reproductive Endocrinology, Surgertand Technology. Philadelphia: Lippincott-Raven; 1996:212-34. |
|
Stouffer RL, Xu F, Duffy DM. Molecular control of ovulation and luteinization in the primate follicle. Frontiers in bioscience : a journal and virtual library 2007;12:297-307. |
|
Kim J, Bagchi IC, Bagchi MK. Signaling by hypoxia-inducible factors is critical for ovulation in mice. Endocrinology 2009;150:3392-400. |
|
Abbott J. Transcervical sterilization. Current opinion in obstetrics & gynecology 2007;19:325-30. |
|
Abbott J. Transcervical sterilization. Best practice & research Clinical obstetrics & gynaecology 2005;19:743-56. |
|
Richart R. Female Sterilization using pharmacologically active agents. In: Zatuchni GI LMSJ, ed. Research Frontiers in Fertility Regulation. Hagerstown: Harper & Row; 1980:262-9. |
|
Alpizar F. Quinacrine sterilization (QS) in Costa Rica: 694 cases. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 2003;83 Suppl 2:S141-5. |
|
Jensen JT, Rodriguez MI, Liechtenstein-Zabrak J, Zalanyi S. Transcervical polidocanol as a nonsurgical method of female sterilization: a pilot study. Contraception 2004;70:111-5. |
|
Jha RK, Jha PK, Guha SK. Smart RISUG: a potential new contraceptive and its magnetic field-mediated sperm interaction. International journal of nanomedicine 2009;4:55-64. |
|
Hafez ES. Function of the fallopian tube in human reproduction. Clinical obstetrics and gynecology 1979;22:61-79. |
|
Coutinho EM, Maia H, Nascimento L. The response of the human Fallopian tube to ergonovine and methyl-ergonovine in vivo. American journal of obstetrics and gynecology 1976;126:48-54. |
|
Saridogan E, Djahanbakhch O, Puddefoot JR, et al. Angiotensin II receptors and angiotensin II stimulation of ciliary activity in human fallopian tube. The Journal of clinical endocrinology and metabolism 1996;81:2719-25. |
|
Abramowicz JS, Archer DF. Uterine endometrial peristalsis--a transvaginal ultrasound study. Fertility and sterility 1990;54:451-4. |
|
Glasier A. Emergency postcoital contraception. The New England journal of medicine 1997;337:1058-64. |
|
Chez R. Emergency oral contraception. ACOG Practice Bulletin 2001:1-6. |
|
Donaldson MS DL, Brown SS, et al. Clinical Applications of Mifepristone (RU486) and Other Antiprogestins. Clinical Applications of Mifepristone (RU486) and Other Antiprogestins,Institute of Medicine. Washington DC: National Academy Press; 1993:1-13. |
|
Lakha F, Ho PC, Van der Spuy ZM, et al. A novel estrogen-free oral contraceptive pill for women: multicentre, double-blind, randomized controlled trial of mifepristone and progestogen-only pill (levonorgestrel). Human reproduction 2007;22:2428-36. |
|
Ramey JW, Starke ME, Gibbons WE, Archer DF. The influence of pentoxifylline (Trental) on the antifertility effect of intrauterine devices in rats. Fertility and sterility 1994;62:181-5. |
|
Grimes DA, Lopez LM, Manion C, Schulz KF. Cochrane systematic reviews of IUD trials: lessons learned. Contraception 2007;75:S55-9. |
|
Ratsula K, Toivonen J, Lahteenmaki P, Luukkainen T. Plasma levonorgestrel levels and ovarian function during the use of a levonorgestrel-releasing intracervical contraceptive device. Contraception 1989;39:195-204. |
|
Nelson A, Apter D, Hauck B, et al. Two low-dose levonorgestrel intrauterine contraceptive systems: a randomized controlled trial. Obstetrics and gynecology 2013;122:1205-13. |
|
El Mahgoub S. Long-term intracervical contraception with a levonorgestrel device. Contraception 1982;25:357-74. |
|
Pakarinen PI, Suvisaari J, Luukkainen T, Lahteenmaki P. Intracervical and fundal administration of levonorgestrel for contraception: endometrial thickness, patterns of bleeding, and persisting ovarian follicles. Fertility and sterility 1997;68:59-64. |
|
Billings EL, Brown JB, Billings JJ, Burger HG. Symptoms and hormonal changes accompanying ovulation. Lancet 1972;1:282-4. |
|
Wade ME, McCarthy P, Abernathy JR, Harris GS, Danzer HC, Uricchio WA. A randomized prospective study of the use-effectiveness of two methods of natural family planning: an interim report. American journal of obstetrics and gynecology 1979;134:628-31. |
|
A prospective multicentre trial of the Ovulation method of natural family planing. II. The effectiveness phase. Fertility and sterility 1981;36(5):591-8. |
|
Coffey PS, Kilbourne-Brook M. Wear and care of the SILCS diaphragm: experience from three countries. Sexual health 2010;7:159-64. |
|
Mauck C, Callahan M, Weiner DH, Dominik R. A comparative study of the safety and efficacy of FemCap, a new vaginal barrier contraceptive, and the Ortho All-Flex diaphragm. The FemCap Investigators' Group. Contraception 1999;60:71-80. |
|
Mauck C, Glover LH, Miller E, et al. Lea's Shield: a study of the safety and efficacy of a new vaginal barrier contraceptive used with and without spermicide. Contraception 1996;53:329-35. |
|
Schwartz JL, Barnhart K, Creinin MD, et al. Comparative crossover study of the PATH Woman's Condom and the FC Female Condom. Contraception 2008;78:465-73. |
|
Madden T, Blumenthal P. Contraceptive vaginal ring. Clinical obstetrics and gynecology 2007;50:878-85. |
|
Baum MM, Butkyavichene I, Gilman J, et al. An intravaginal ring for the simultaneous delivery of multiple drugs. Journal of pharmaceutical sciences 2012;101:2833-43. |
|
Johnson TJ, Clark MR, Albright TH, et al. A 90-day tenofovir reservoir intravaginal ring for mucosal HIV prophylaxis. Antimicrobial agents and chemotherapy 2012;56:6272-83. |
|
Norton EJ, Diekman AB, Westbrook VA, et al. A male genital tract-specific carbohydrate epitope on human CD52: implications for immunocontraception. Tissue antigens 2002;60:354-64. |
|
Xu B, Hao Z, Jha KN, et al. Validation of a testis specific serine/threonine kinase [TSSK] family and the substrate of TSSK1 & 2, TSKS, as contraceptive targets. Society of Reproduction and Fertility supplement 2007;63:87-101. |
|
Diekman AB, Herr JC. Sperm antigens and their use in the development of an immunocontraceptive. American journal of reproductive immunology 1997;37:111-7. |
|
Kurth BE, Digilio L, Snow P, et al. Immunogenicity of a multi-component recombinant human acrosomal protein vaccine in female Macaca fascicularis. Journal of reproductive immunology 2008;77:126-41. |
|
Naz RK. Contraceptive vaccines: success, status, and future perspective. American journal of reproductive immunology 2011;66:2-4. |
|
Naz RK, Sacco A, Singh O, Pal R, Talwar GP. Development of contraceptive vaccines for humans using antigens derived from gametes (spermatozoa and zona pellucida) and hormones (human chorionic gonadotrophin): current status. Human reproduction update 1995;1:1-18. |
|
Naz R, Aleem A. Effect of immunization with six sperm peptide vaccines on fertility of female mice. Society of Reproduction and Fertility supplement 2007;63:455-64. |
|
Goldberg E. Current status of research on sperm antigens: potential applications as contraceptive vaccines. Research frontiers in fertility regulation : RFFR / PARFR 1983;2:1-11. |
|
O'Rand MG LJ. Update on contraceptive vaccines; understanding the immumne response. In: - JoRI, ed. Infertil Reprod Med Clin N America: Elsevier; 2002:221. |
|
Sacco AG. Immunocontraception: consideration of the zona pellucida as a target antigen. Obstetrics and gynecology annual 1981;10:1-26. |
|
Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C. Severely reduced female fertility in CD9-deficient mice. Science 2000;287:319-21. |
|
Naz RK. Contraceptive vaccines. Drugs 2005;65:593-603. |
|
Lane VM, Liu IK, Casey K, et al. Inoculation of female American black bears (Ursus americanus) with partially purified porcine zona pellucidae limits cub production. Reproduction, fertility, and development 2007;19:617-25. |
|
Naz RK, Gupta SK, Gupta JC, Vyas HK, Talwar AG. Recent advances in contraceptive vaccine development: a mini-review. Human reproduction 2005;20:3271-83. |
|
Talwar GP. Making of a vaccine preventing pregnancy without impairment of ovulation and derangement of menstrual regularity and bleeding profiles. Contraception 2013;87:280-7. |